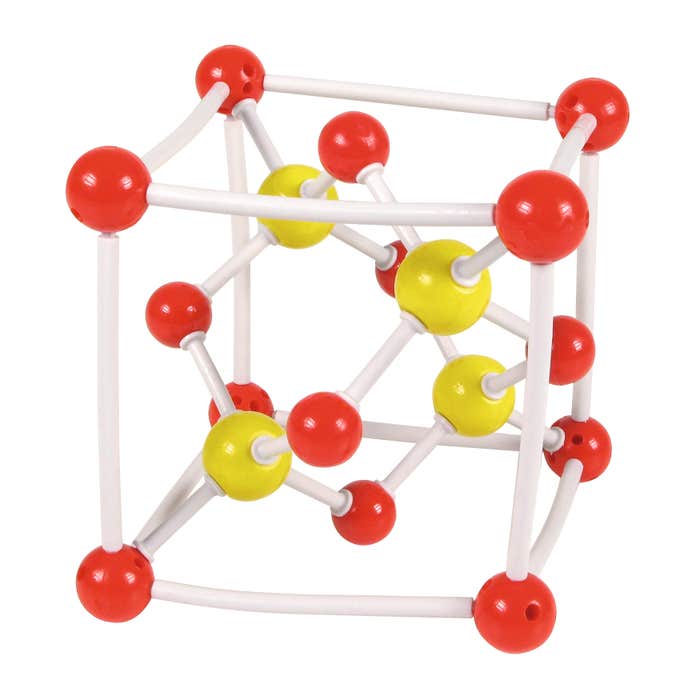
Crystal structure of Zinc Sulfide
Sku: 100181
€26.33
€31.60
Exploded view of a classic face-centered cubic lattice of one of the elements (Zinc), where four out of the eight tetrahedral sites (sites located between an atom at a lattice corner and the atoms at the centers of the three intersecting faces) are occupied by atoms of another element (in this example, sulfur).
This crystalline form gave its name to the so-called 'blende' form.
14 Zinc atoms, 4 Sulfur atoms, 28 bonds, to assemble.
| Type de modèle | Chimie structurale |
|
Caractéristiques techniques
Dimensions: 15 x 15 x 15 cm
|